Wu Yusen, Luo Jing, has the same disease but different lives, fighting against the "chameleon" lymphoma in the tumor field, and the new anti-cancer force CAR-T rises!
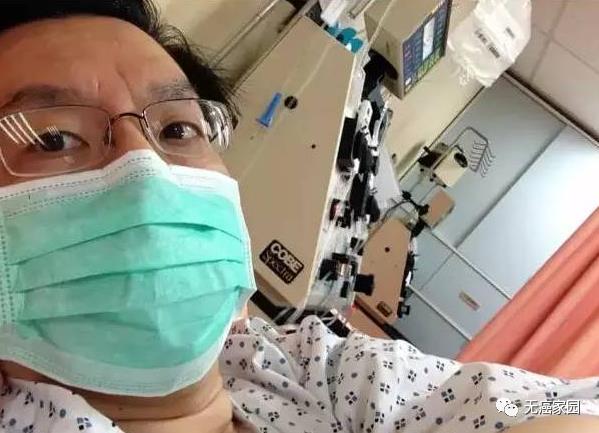
Luo Jing, the host of the famous News Network, Cindy Lee, the actor who plays Fang Yu in Deep Rain, and Fuck off! Tumor jun touched many netizens’ cartoonist Xiong Dun … In recent years, many celebrities have passed away because of lymphoma. At the same time, however, there are also many famous people who have succeeded in fighting cancer after suffering from lymphoma. For example, the 70-year-old famous director Wu Yusen was diagnosed with large B-cell lymphoma in tonsils when filming Taiping Wheel in 2008. After four operations, in the second month of chemotherapy, his mouth was all festered, he could not swallow, and his body and face were so thin that he was wrinkled. Finally, he succeeded in fighting cancer with his firm will!

For example, Kai-Fu Lee, the founder of an innovative factory that successfully used chemotherapy and targeted therapy to fight advanced lymphoma! On September 5, 2013, 52-year-old Kai-fu Lee announced that he had stage IV follicular lymphoma and had to leave work for treatment. In February 2015, he announced the results of his physical examination, indicating that the tumor in the body is not obvious at present. In June of the same year, Kai-fu Lee said that he had not found any lesions in his last two examinations and ridiculed himself as "Li Kangfu". It took Kai-fu Lee less than two years from finding out the cancer until the focus was completely gone. His anti-cancer experience is worth learning from.
Alias "the most disguised disease", lymphoma has become a killer.
According to statistics, there is a new case of lymphoma every 9 minutes in the world. The incidence of lymphoma in China is 0.02‰, with about 25,000 new patients and nearly 20,000 deaths each year. The threat of lymphoma is rapidly emerging. It ranks among the top ten malignant tumors in China and ranks second among hematological malignancies. At the beginning, the symptoms of the disease were hidden, and it was described as "the most disguised disease". In the intercontinental area, the incidence of non-Hodgkin’s lymphoma is much higher than Hodgkin’s disease, which is about 9: 1. Because of the higher degree of malignancy and worse prognosis, lymphoma is generally referred to as non-Hodgkin’s lymphoma. In China, the incidence of malignant tumors ranks ninth among men and tenth among women. Non-Hodgkin’s lymphoma can occur in all ages, and the high incidence age is 45~60 years old. Among them, Luo Jing suffered from diffuse large B-cell lymphoma (DLBCL). This is a common non-Hodgkin’s lymphoma, which belongs to aggressive lymphoma with high malignancy. The course of the disease progresses rapidly. If it is not actively treated, the median survival time is less than one year. In recent years, great progress has been made in the research and treatment of lymphoma, and new drugs and technologies have risen strongly! CAR-T therapy Among them, the chimeric antigen receptor T(CAR-T) cell therapy is particularly prominent. Its appearance represents a great progress in personalized cancer treatment, especially bringing the hope of rebirth to patients with hematological malignancies.
Complete remission! The first advanced lymphoma patient in China who received CAR-T therapy was discharged smoothly!
Although they all suffer from diffuse large B-cell lymphoma, Mr. Chen, who suffers from malignant lymphoma, is a lucky one. According to Jiangsu public news channel News Space Station, on August 31st, the first lymphoma patient treated with CAR-T cells in China achieved complete remission and was discharged smoothly.

Mr. Chen suffered from diffuse large B-cell lymphoma. After the onset of the disease in October 2010, he had received chemotherapy for many times, but all of them were unsatisfactory. Diffuse large B-cell lymphoma is the most common malignant lymphoma. After immunotherapy, about 60% patients can survive for a long time, but 40% patients will still relapse. In June this year, Mr. Chen accepted CAR-T cell therapy with a tentative attitude, and all the physical indicators tended to be normal. He told reporters with delight that his physical condition was better than before, and he was no different from a normal person after leaving the hospital. CAR-T therapy, a "living drug", made her completely relieved and looked forward to a cancer-free future.
As a "living" drug, CAR-T therapy is very different from traditional drugs. First, the therapy needs to isolate T cells from patients and modify them with chimeric antigen receptor (CAR) in vitro, so that they can specifically recognize cancer cells. Finally, the modified T cells are expanded and reinfused into patients. Compared with traditional chemotherapy and hematopoietic stem cell transplantation, it can kill tumor cells more accurately, improve the curative effect and greatly reduce the side effects.
The well-known director of hematology in China said: CAR-T can identify lymphoma very deliberately, and the antigen carried on tumor cells will attack directly after being connected, and it will produce an anti-tumor effect and kill it directly without the need for second signal stimulation, so one of its characteristics is accuracy, and the second is power. The scope of application is still the recurrence or refractory diffuse large B lymphoma that is ineffective in general immunochemotherapy.
CAR-T therapy FDA approved 6 models, and domestic CAR-T therapy caught up!
After sharing the above news reports about CAR-T therapy, let’s get down to business and talk about CAR-T therapy. At present,CAR-T therapy has achieved great success in the treatment of hematological malignancies.. Six CAR-T cell therapies have been approved by FDA in the United States and two CAR-T cell therapies have been listed in China. The picture below shows CAR-T cell therapy that has been listed at home and abroad. 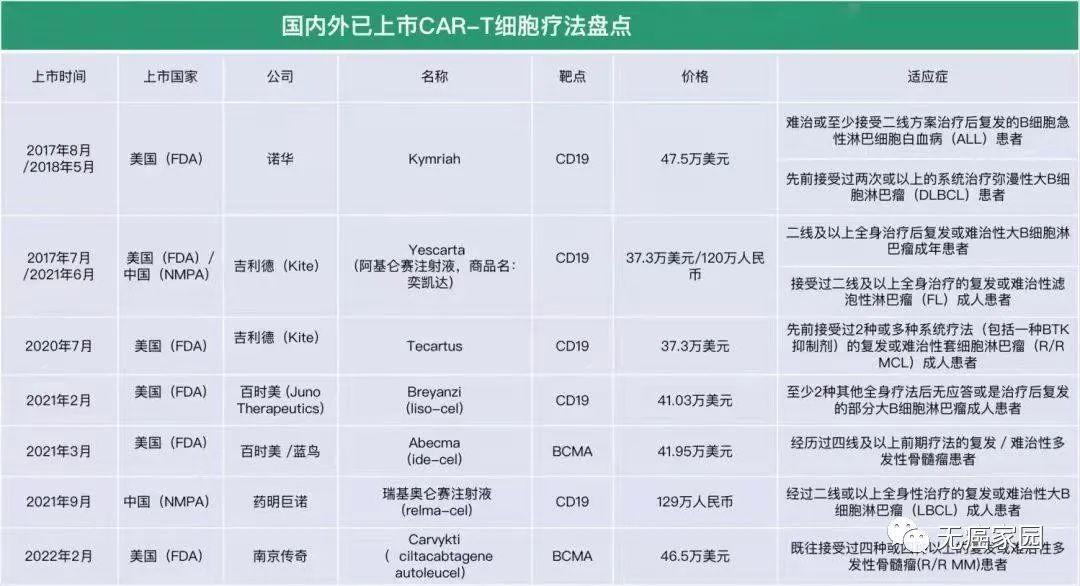
Although China’s CAR-T technology started late, the domestic market is large. According to the data on Clinicaltrials.gov’s website, China became the country that registered the most CAR-T tests in September 2017. As of May 27th, 2021, there are 1301 CAR-T clinical trials in the world, and the number of CAR-T trials registered in China has reached 478!
01 The first CAR-T therapy in China was approved for listing! China welcomes the first year of cellular immunotherapy.
On June 22nd, the blank in the field of CAR-T in China was finally broken! The latest announcement of China National Medical Products Administration (NMPA), Fosun Kaite Biotechnology Co., Ltd. (hereinafter referred to as " Fosun Kate ”) The CAR-T cell therapy product targeting CD19, Aquilencel Injection (also known as Aquilencel, FKC876), was officially approved for marketing. This is the first approved CAR-T therapy in China and the sixth approved CAR-T therapy in the world. It is believed that China will usher in the blowout era of CAR-T cell therapy.
02 In just one month, the second CAR-T therapy in China and the sixth CAR-T therapy in the world were approved!
On September 3, the State Drug Administration recently announced that Yaoming JunuoThe CAR-T product targeting CD19, Regiosai Injection.(relma-cel, trade name: Benoda) has been officially approved. The approved indications for Regioside Injection are:It can be used to treat relapsed or refractory large B-cell lymphoma (r/r LBCL) in adult patients after second-line or above systemic treatment.Reggiorense injection is the second CAR-T product approved in China and the first CAR-T product of Class 1 biological products in China.

It’s only been about one month since it was submitted for examination and approval on July 30th last time, which can be described as rapid progress. Congratulations! (At present, patients with economic conditions can contact cancer-free homes for the treatment of regiorense. )
Boom! The first BCMA CAR-T therapy in China and the second in the world was approved by FDA!
China’s "dark horse" in CAR-T cell therapy industry-legendary BCMA(B-cell mature antigen) CAR-T therapy Cedar-B38m/JNJ-4528 (hereinafter referred to as cilta-cel) has attracted much attention, and has shown excellent anticancer activity in clinical trials for multiple myeloma.
What is exciting is that on February 28th, 2022, local time, it was jointly developed by Janssen and Legendary Biology.BCMA CAR-T product, Cedactylosin (trade name Carvykti), has been approved by the US FDA for the treatment of adult patients with relapsed/refractory multiple myeloma (MM).
This is the first cell therapy product approved by FDA in China and the second CAR-T cell immunotherapy approved for BCMA in the world.

Cilta-cel was awarded priority review qualification by FDA on May 26th.However, the approval of the West Dachiorensai was delayed, and it was adjusted from November 29, 2021 toFebruary 28, 2022Fortunately, it was finally approved and lived up to expectations!
cilta-celIt is a CAR-T therapy directed by research B cell maturation antigen (BCMA), which is used to treat relapsed or refractory multiple myeloma (RRMM). These patients received an average of five kinds of pre-treatment before treatment, 76% of them received five kinds of treatment, and 86% of them were resistant to five kinds of treatment, including CD38 treatment, and they were both resistant to protease inhibitor PI and immunomodulator IMiD. Simply put, it is refractory to relapse and lack of effective treatment in the follow-up. The principle of this CAR-T therapy is different from the current PD-1/L1. It directly uses the T cells in the patient’s body, transforms them in vitro, replicates them in large quantities, and then transfuses them into the patient’s body to kill cancer cells. At the ASH conference in 2021, the latest results of the 1b/2 CARTITUDE-1 study (n=97) showed that,meso-position 22 months Long-term follow-up, The objective remission rate (ORR) reached 98% , 83% patients achieved strict complete remission (sCR) It is emphasized that the mitigation will deepen with the passage of time (sCR will increase from 67% reported at the ASH annual meeting in 2020 to 83%). At 18 months, 66% of the patients were alive and the disease did not progress. .The two-year progression-free survival rate and overall survival rate are respectively 61% and 74% .The latest research results to be announced at the ASCO annual meeting in 2021 show that After a median follow-up of 18 months, the overall survival (OS) rate was 81%. The remission rate is comparable in all pre-specified subgroups and patients with different treatment lines.
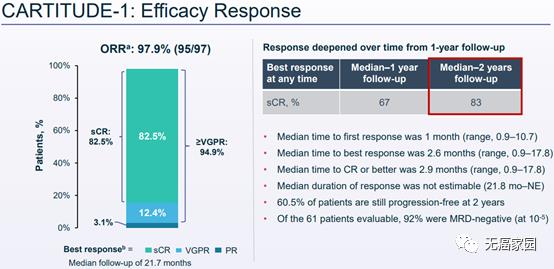
American research shows that cilta-cel has excellent long-term efficacy.
Emily, a foreign country, successfully ushered in nine years of cancer-free survival in 2021, and suddenly became the "spokesperson" of global CAR-T therapy. In China, there is such a lucky person whose fate has been completely changed by CAR-T therapy. Mr. Min was diagnosed with a special rare disease at the age of 54- multiple myeloma . He had no drugs available at that time, so he was lucky enough to participate in the clinical trial of CAR-T therapy. Although the treatment process was not smooth sailing, after three times of treatment, all the lumps on Mr. Min’s body disappeared and the pain disappeared. Surprisingly, by June 4, 2021, Mr. Min had lived cancer-free for five years, which is the field of cancer treatment, which is theoretically said. Clinical cure .
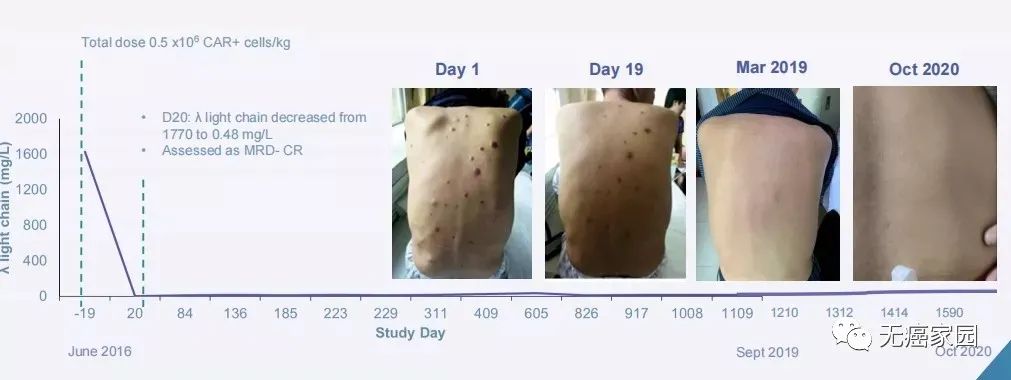
In fact, as early as March 27th this year,The FDA approved the first CAR-T therapy targeting BCMA for adult patients with relapsed/refractory multiple myeloma (r/r MM) after receiving more than four therapies.
Chemotherapy to fight cancer is often "killing one thousand enemies and losing eight hundred". Antibody drugs can accurately find tumor targets, but sometimes their combat effectiveness is insufficient. If we can put a "warhead" of chemotherapy drugs on antibody drugs, can we attack cancer cells more accurately? Based on this, an ADC (antibody drug conjugate) called "biological missile" was born. Antibody-coupled drugs are like biological missiles, which consist of two core functions: the first is antibody (missile body), the second is strong chemotherapy drug (nuclear warhead); This design will enable antibodies to find tumor cells with chemotherapy drugs, and then accurately poison tumor cells and poison them. This drug design combines the precision of targeted drugs with the high efficiency of chemotherapy drugs, which not only avoids the toxic and side effects of systemic use of chemotherapy drugs, but also has stronger killing ability than using targeted drugs alone, killing two birds with one stone. 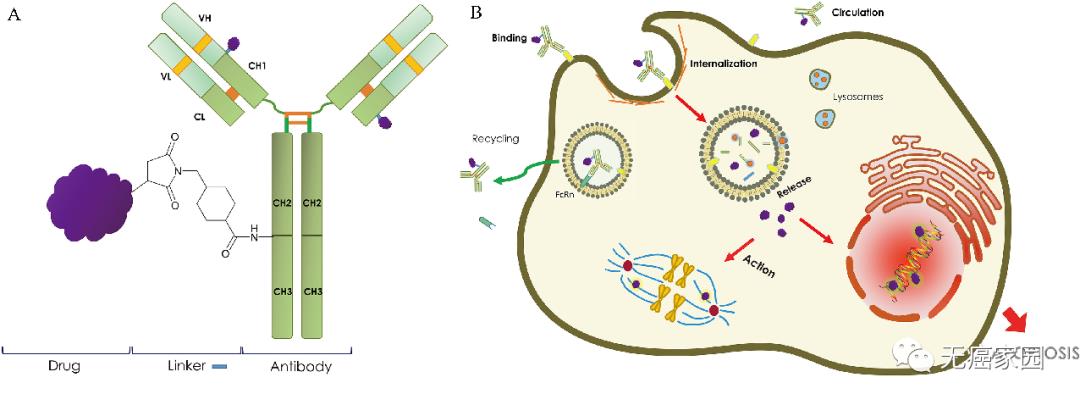
Schematic diagram of antibody-coupled drugs Note: (a) General description of A)ADC structure; (b) the mechanism of B)ADC.
Fourteen ADC drugs have been approved around the world, which brings hope for prolonging the survival time of back-line patients.
At present, more than 100 kinds of ADC are undergoing clinical trials. Most adcs have progressed from phase i to phase II. Some ADC phase III trials show positive results; Up to September 20th, 2021, there are 14 kinds of ADC drugs on the market in the world, which are mainly used in hematological tumors and solid tumors, and are mainly used for back-line treatment of patients, including advanced, recurrent/refractory and metastatic tumor indications.
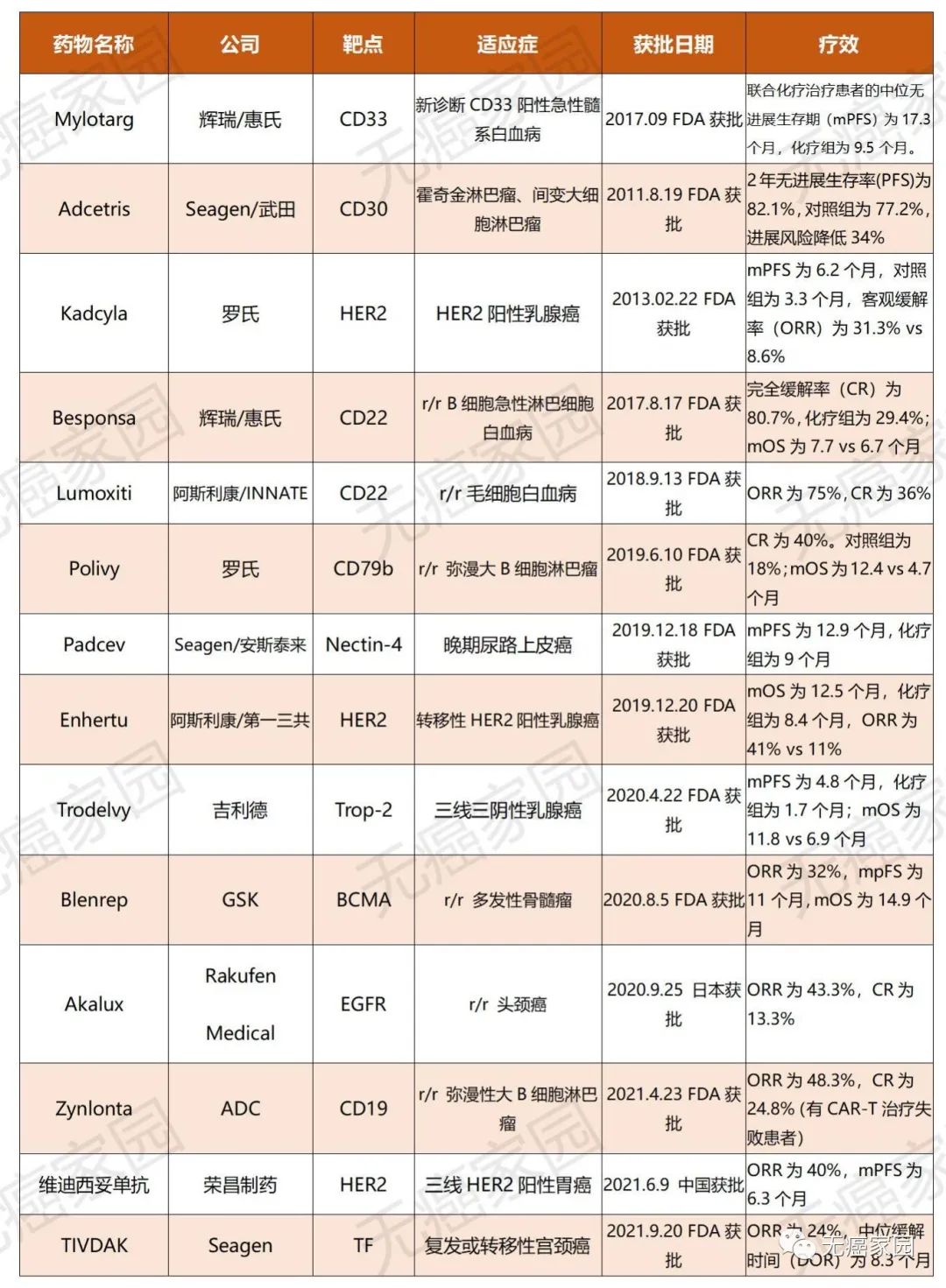
Fourteen ADC drugs have been approved worldwide.
According to the curative effect data, ADC drugs have brought more treatment options and the hope of prolonging the survival time for back-line patients, and the objective remission rate or median progression-free survival time of several drugs has doubled compared with chemotherapy.
Looking at the CD30 target, the new drug F0002-ADC became an instant hit and squeezed into the lymphoma track.
CD30, a member of tumor necrosis factor (TNF) receptor superfamily, can promote cell proliferation or apoptosis by activating different signal pathways. In 2020, Vebtuximab for injection (Adcetris) was approved to be listed in China, and CD30 became famous. Adcetris, as an antibody-coupled drug targeting CD30, has an effective rate of 73% for Hodgkin’s lymphoma (HL) and 86% for anaplastic large cell lymphoma (ALCL). It is the first new drug approved in the field of Hodgkin’s lymphoma treatment in recent 30 years, and it is also the first time that a drug targeting CD30 has been approved, filling the gap in this field. At present, the only new ADC drug for CD30 in China is F0002-ADC. This new drug consists of three parts, namely, human-mouse chimeric anti-CD30 monoclonal antibody, thioether linker (MCC) and DM1. Since the project was launched in 2016, it has been disappointing! At present, patients with recurrent/refractory CD30 positive peripheral T-cell lymphoma are being recruited clinically! This is a new treatment option for patients with recurrent/refractory peripheral T-cell lymphoma!
This article is original for a cancer-free home, and it needs authorization to reprint! Cancer-free homes remind patients